With much more - and higher quality - data, scientists believe that computer models can now design more targeted and thus effective vaccines, which could shorten response times and reduce both illness and its severity going forward. JL
Elie Dolgin reports in Nature, image Institute for Protein Design:
A growing number of scientists think that the industry, with an eye towards optimizing immune responses, (can) develop mathematical and computational models to inform dose decision-making for vaccine trials. Researchers modelled dosing schemes, prioritizing population-level immunity in one scenario, individual immunogenicity and safety in another and factoring in cost containment. In every situation, the optimal dose was more than double the amount now approved for use in China and elsewhere. “We’re in a position now where, going forward, we can be much more intentional about vaccine design.”When Moderna joined the hunt for a coronavirus vaccine in early 2020, the company had only limited clinical experience with its technology.
Scientists had tested the company’s messenger RNA (mRNA)-based vaccines against a few viruses, such as avian influenza and Zika, in humans. They found that the highest dose levels — upwards of 300 micrograms — often triggered undesirable side effects. The lowest doses (around 10 µg) did not always elicit a sufficient immune response.
There seemed to be a happy medium: in a two-dose vaccine for another respiratory virus with pandemic potential1, a new strain of bird flu, the sweet spot was around 100 µg. So, it made intuitive sense for Moderna, based in Cambridge, Massachusetts, and its collaborators at the US National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland, to try something similar to tackle SARS-CoV-2.
Within days of confirming that the coronavirus vaccine it had developed offered protection in mice, the company started human trials, testing doses of 100 µg to see whether its intuition was right. It also trialled 25- and 250-µg doses in case it wasn’t.
“The whole point was to go quickly,” says Barney Graham, former deputy director of the NIAID’s Vaccine Research Center, who oversaw the vaccine’s early development. “That’s just how it’s done. It’s not a real precise process.”
Other efforts to develop COVID-19 vaccines followed a similar playbook. The University of Oxford, UK, in partnership with AstraZeneca in Cambridge, UK, started testing its vaccine — made from an engineered adenovirus — at a dose of 50 billion viral particles. It chose the dose in large part because that amount was used in previous trials of the same vaccine platform for other pathogens, including the coronavirus responsible for Middle East respiratory syndrome.
This approach to dose selection produced several safe and effective COVID-19 vaccines in record time — which has helped to save millions of lives around the world — but it did not necessarily take full advantage of the vaccines’ pandemic-altering potential, scientists say. Some blame companies’ inexact, educated guesses for a high rate of adverse events connected to many shots, the diminished efficacy of others and several high-profile trial failures, including initial attempts to develop a shot for young children.
“It still feels like people just threw a lot of spaghetti at the wall and saw what stuck,” says Thomas Evans, chief scientific officer of Vaccitech in Oxford, which devised the adenovirus-mediated gene-delivery system found in the Oxford–AstraZeneca vaccine.
A growing number of scientists think that the industry can do better. With an eye towards optimizing immune responses, they have been developing mathematical and computational models over the past several years to inform dose decision-making for vaccine trials. Not everyone is convinced the models are ready for prime time; many aren’t even aware that the platforms exist. But those who embrace the technology say that, if companies had simply capitalized on all the tools at their disposal, COVID-19 vaccines might be doing an even better job at containing viral spread and limiting collateral damage. “We missed a huge opportunity,” Evans says.
Leap of faith
Pharmaceutical companies have long used computational modelling strategies to fine-tune drug dosing, but such techniques have rarely been applied to vaccine development. Past experience and animal testing typically guide dose selection for experimental vaccines — and things were no different for those against COVID-19. The result was a range of doses for the products (see ‘Dosing decisions’).
Vaccine developers chose amounts that worked in other disease contexts, but immune responses can vary widely from one pathogen to the next. Animal studies add a degree of confidence in a vaccine’s success — but the immune system of a mouse or monkey is not the same as that of a human, and scientists don’t fully understand how to scale doses across species. So, most vaccine companies simply made what Jeff Barrett, a quantitative pharmacologist at the Critical Path Institute, a non-profit organization in Tuscon, Arizona, describes as “a leap of faith” from animal models to human testing.
Moderna’s vaccine programme was no exception. Researchers involved in the earliest mouse studies administered two-shot regimens, with doses of up to 20 µg each2. But, according to Graham, they made little attempt to quantitatively map the immune responses observed in mice that received different doses to anticipated outcomes in people: the plan all along was to anchor human trials around the 100-µg dose that worked best for Moderna’s bird-flu vaccine candidate.
Company executives defend the approach because of the time and data constraints. “You make the best decision you can,” says Jacqueline Miller, head of infectious diseases at Moderna, “but that is informed by some of the previous programmes that had small phase I data with other vaccine antigens.”
Business considerations factor in as well. Pfizer in New York City and BioNTech in Mainz, Germany, opted for a shorter gap between doses for their mRNA jab, in part to help them beat Moderna in the race to marketing authorization, whereas Johnson & Johnson in New Brunswick, New Jersey, initially advanced a one-dose regimen to differentiate the company’s COVID-19 vaccine from others in development.
There were also public-health arguments for these dosing decisions, such as getting people vaccinated quickly. And the choices made during the sprint to provide a vaccine had real-world consequences.
Moderna’s 100-µg shot proved to offer greater protection against infection, disease and hospitalization than the one from Pfizer–BioNTech, which used only 30 µg of mRNA per injection. By one estimate, recipients of the Pfizer–BioNTech vaccine had a 58% greater risk of infection from the Delta variant of SARS-CoV-2 than those who got Moderna’s shot3. (The trade-off is that Moderna’s product came with more frequent post-vaccine reactions.)
Differences in formulation and administration schedules could be at play, says John Moore, an immunologist at Weill Cornell Medicine in New York City. But he, like many researchers, points to dose size as the most probable explanation for differences in efficacy and tolerability. Moderna’s own head-to-head trials of 50- and 100-µg dosing schemes support this conclusion4.
Perhaps a more consequential decision was the one that executives at Pfizer and BioNTech made for their child-sized COVID-19 vaccine.
In a small trial involving a few dozen children under 5 years old, the companies found that a pair of 3-µg shots was sufficient to prompt an antibody response comparable to that in teenagers and young adults who had received two full doses. What’s more, the mini-dose didn’t trigger the severe fevers observed among children given a 10-µg shot, so Pfizer and BioNTech moved ahead with the smallest dose.
But in a later-stage trial involving thousands of infants and young children, protection came up short. Two- to four-year-olds failed to produce enough antibodies, and a third booster might be necessary to develop adequate immune protection in those children.
Meanwhile, Moderna announced last month that a 25-µg version of its shot provided the same level of immune protection against COVID-19 in children under six, as did a full 100-µg dose in young adults. Moderna is now moving forwards with global regulatory submissions in every age bracket.
Tricky business
With the benefit of hindsight, most scientists now think that Pfizer and BioNTech chose too low a dose for children under five. But it’s hard to fault the thinking of drug executives, who were trying to minimize side effects, says Karim Azer, who has previously worked on tuberculosis-vaccine modelling at the Bill & Melinda Gates Medical Research Institute in Cambridge, Massachusetts. “The dose–response relationship with vaccines can be very tricky,” he says.
There are several complicating factors. With conventional pharmaceuticals, greater drug concentrations usually yield more potent effects, at least up to a certain level. This isn’t the case with vaccines, because higher doses can sometimes produce less favourable responses.
That’s because repeat exposure to vaccine antigens can cause certain arms of the immune system to secrete enough pro-inflammatory signalling molecules to trigger a phenomenon known as immune exhaustion, leading to impaired protection.
Timing is also important: a long interval between shots might coax out more protective antibodies, but one that’s too long risks missing an optimal window. And dose–response dynamics often differ widely by age. A child is not simply a small adult when it comes to vaccines — more so than for other medicines.
Then, there’s the question of what to measure when calculating vaccine-mediated protection. Antibodies or immune cells? Rates of infection or of disease and death?
“A definition of optimal dose may vary depending on which of these factors you care about,” says John Benest, a mathematical biologist at the London School of Hygiene & Tropical Medicine (LSHTM).
Together with his supervisor Richard White and former group member Sophie Rhodes, Benest took published data from an early clinical study sponsored by CanSino Biologics in Tinjian, China, maker of a one-shot COVID-19 vaccine based on a viral vector. The researchers modelled5 dosing schemes, prioritizing population-level immunity in one scenario, individual immunogenicity and safety in another and factoring in cost containment in a third. In every situation, the optimal dose — predicted on the basis of data from CanSino’s first-in-human trial — was more than double the amount now approved for use in China and elsewhere.
“It’s a shame,” says Rhodes, now a staff scientist at Certara, a drug-development consultancy headquartered in Princeton, New Jersey. Had she and other specialists in infectious-disease modelling been better positioned to obtain those kinds of quantitative insight early in the pandemic response, “it could have changed the way that we developed vaccines”, Rhodes says. CanSino chief scientific officer Tao Zhu stands by the company’s dosing decisions: the LSHTM analysis “is a good model”, he says, but it doesn’t account for logistical challenges in administering higher doses and later-stage trial data that shaped the company’s final call on which dose to use.
Model makers
The LSHTM researchers, along with Evans and others, have been at the forefront of efforts to create a mathematical framework for informing vaccine dose decision-making6. In 2015, they convened the world’s first workshop dedicated to the topic. (Only a few dozen people attended.) In the years since, they have honed their modelling techniques with an eye to streamlining the process of determining vaccine doses for early-stage trials.
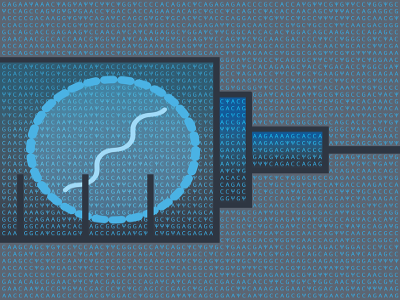
For any vaccine candidate, the modelling framework starts with data. The researchers feed immune-response results from animal experiments into their equations to produce a predicted dose–response curve. They then scale that dose–response relationship to humans using clinical data from a more limited number of doses, often from historical work on similar vaccines. In this way, they come up with expected ‘best’ doses for testing in human trials — and they can further refine the model’s predictions as more data become available (see ‘Immune modelling’).
As a proof of principle, the researchers fit their model with mouse and human data on the response of T cells — a type of immune cell — to an experimental tuberculosis vaccine. The mathematics then predicted that lower doses would offer the best immune response7. Independent clinical studies run in parallel to the group’s modelling project bore this out.
Jennifer Linderman, a systems biologist at the University of Michigan in Ann Arbor, says such approaches could be useful for guiding dose decisions in the future. “We’re in a position now where, going forward, we can be much more intentional about vaccine design,” she says. She and Denise Kirschner, a computational immunologist at the University of Michigan Medical School, developed HostSim, a model that incorporates lung biology alongside simulations of what happens in the blood and lymph nodes8.
Although initially focused on tuberculosis, Kirschner notes that, with the appropriate data inputs, her team’s tool could guide vaccine development for any pathogen that infects the airways. “We can use our model for flu. We can use our model for COVID. We can use it for lots of things,” she says.
In simulated trials, at least, such modelling approaches allow vaccine developers to vet many more doses than would otherwise be feasible, even in the largest of clinical studies. “You can test a wider range of scenarios in silico,” says Luca Marchetti, a computer scientist at the Microsoft Research–University of Trento Centre for Computational and Systems Biology in Rovereto, Italy, who last year developed a model to support mRNA-vaccine development9.
Pandemic response
Before the pandemic, few companies wanted to invest in this kind of vaccine-dose modelling. Around five years ago, Evans and Kent Kester, then head of translational sciences at Sanofi Pasteur (the vaccines division of Paris-based Sanofi), tried to create a research consortium focused on tool development in this area, but they failed to get buy-in from industry or regulatory authorities. “No one was really interested in pursuing it,” says Kester, now vice-president of translational medicine at IAVI, a vaccine non-profit organization based in New York City.
Because of COVID-19, more vaccine manufacturers are now experimenting with dose modelling, and regulatory agencies are monitoring the science closely.
“This is an important area,” says Marco Cavaleri, head of biological health threats and vaccines strategy at the European Medicines Agency in Amsterdam. “The more we can refine these techniques, the more we will be prepared in the future.”
Last June, the US Food and Drug Administration convened a workshop at which researchers discussed best practices for modelling vaccine dose–response relationships. White and Rhodes spoke, as did Andrzej Kierzek and Piet van der Graaf, modellers at Certara who, before the pandemic, had created a tool for running virtual trial simulations of antibody drugs and biological therapies.
The tool had helped pharmaceutical companies to predict unwanted immune reactions. But when COVID-19 hit, the Certara researchers realized that the same model could be used to forecast desired immune responses from vaccines. As a first test, they plugged in the amino-acid sequence corresponding to the coronavirus’s spike protein, the bit used by most COVID-19 vaccinese. As van der Graaf recalls: “We got a surprising, meaningful result.”
The immune responses predicted by the model “seemed to be plausible”, he says. And as companies such as Moderna and Pfizer–BioNTech began to publish more human and mouse data, the Certara scientists would incorporate those results into their simulation workflow. They added response dynamics for T cells and B cells, which produce antibodies, into the mix, along with plug-in modules to account for different vaccine technologies and routes of administration.
Over time, their model — dubbed the Vaccine Simulator — grew in sophistication. And before efficacy results were even known for the first wave of COVID-19 vaccines, Kierzek and van der Graaf had already concluded that longer dosing intervals than those being evaluated would yield improved antibody responses10. Data from the United Kingdom, where extended dosing schedules were routine during the vaccine roll-out, later confirmed that advantage.
Dose decisions
Daiichi Sankyo was one of the first drug companies to incorporate the Certara platform into its vaccine-development programme. The Tokyo-based firm began testing its mRNA vaccine in humans in March 2021 — a slow start that gave trial organizers the opportunity to learn from the experiences of other companies.
Scientists at Daiichi looked at the dosing and scheduling regimens of other mRNA shots, and determined that a dose between the 30 µg used by Pfizer–BioNTech and the 100 µg used by Moderna might provide the ideal balance of immunogenicity and tolerability. They planned to press forwards with an initial trial evaluating up to 60-µg doses of mRNA.
But when the company, in partnership with Certara, simulated immune responses to the vaccine in virtual participants, they found that older individuals failed to mount robust antibody responses at the highest planned dose. “This result triggered an internal discussion on the phase I study design,” says Daiichi’s Ryoko Sawamura, who leads a modelling team at the company. Ultimately, her firm added a 100-µg dose to its first-in-human trial protocol.
AstraZeneca scientists are now using Certara’s model to simulate scenarios not captured by earlier clinical studies of the company’s vaccine. They are interrogating immune responses in populations that were under-represented in trials — particular ethnic groups, for example, and immunocompromised people — to predict who might benefit from non-standard dosing. And they are looking at long-term immunity trends to inform optimal timing of booster-dose regimens.
Few seasoned vaccine developers are converts to the approach. “There are too many variables to model in a way,” says Emilio Emini, chief executive of the Gates Medical Research Institute and a former vaccine research head at Pfizer and Merck. “At the moment, there are no clear prospective models that exist that allow one to make that initial prediction — other than extrapolating as best as one can,” he adds.
But such modelling tactics are catching on. The FDA says that, at the end of 2021, it received its first submission of a vaccine product created using modelling to optimize dose–response relationships.
Although Moderna’s Miller and other industry executives say it’s too soon to begin prospectively selecting vaccine doses for human trials, they might change their minds if and when the tools get validated and prove their worth. “As we gain more experience,” Miller says, “we’ll get there.”







cheap smm panel india smm bear This information is enthralling and inventive. I've been debating whether or not to transfer to a different college, and this has helped me with one part.
ReplyDeleteGuys, can I join your discussion? Until recently, I had the same question, and I decided to look for a tool that would be useful for restoring the balance between rest and work. I finally found this service where I order CBD that helps me feel relaxed and gain strength for further work. I think you can try to use these useful products.
ReplyDelete